In our previous tutorial we discussed Elasticity, Hooke’s law and more, in this tutorial you will learn the following:
- The primary physical characteristics of matter.
- The various phases of matter
- The mechanisms of the various phases of matter.
Introduction
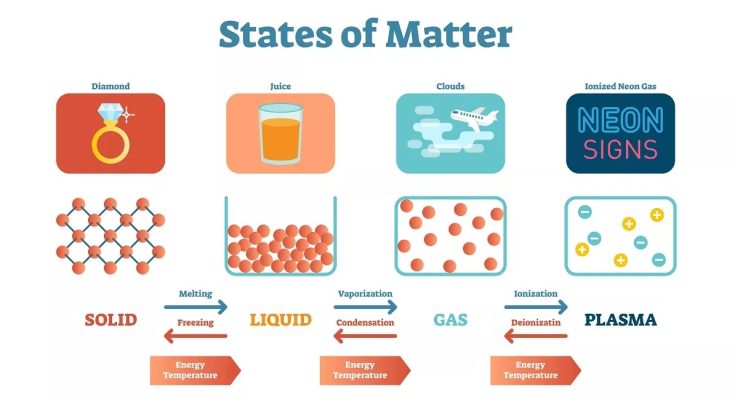
Generally, in our everyday lives, matter is considered to exist in four states which are labelled as:
- Solid state
- Liquid state
- Gaseous state
- Plasma state
There are states of matter that only exists in some extreme conditions; such as the Bose-Einstein Condensates (extreme cold), neutron degenerate (extreme density) and quark-gluon plasma (at extremely high energy).
Here, we will focus more on the four states of matter as listed above.
Physical Characteristics
Physical characteristics refer to the observable properties of matter that can be measured or described without changing the substance’s chemical composition. These characteristics provide information about the nature and behavior of matter.
Common Physical Characteristics of Matter:
- Mass: Mass is a measure of the amount of matter in an object. It is a fundamental property and remains constant regardless of the location of the object. Mass is typically measured in kilograms (kg) or grams (g).
- Volume: Volume refers to the amount of space occupied by an object or substance. It can be measured in cubic units, such as cubic meters (m³) or cubic centimeters (cm³). The volume of a regular object can be determined using mathematical formulas, while irregular objects may require techniques like water displacement.
- Density: Density is the mass per unit volume of a substance. It describes how tightly packed the particles are within a material. Density is calculated by dividing the mass of an object by its volume and is usually expressed in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). It helps identify substances and predict their behavior, such as whether they will sink or float in a particular medium.
- Color: Color refers to the visual appearance of an object or substance due to the way it reflects or absorbs light. The color is determined by the wavelengths of light that are reflected by the material. Different substances can exhibit a wide range of colors, providing visual cues and aiding in identification.
- Odor: Odor is the characteristic smell associated with a substance. Different substances have distinct odors due to their chemical composition. The sense of smell helps in identifying substances and detecting changes, such as spoilage or chemical reactions.
- Texture: Texture refers to the surface characteristics or feel of a substance. It describes qualities such as roughness, smoothness, softness, or hardness. Texture is determined by the arrangement and nature of the particles that make up the material.
- Melting Point: Melting point is the temperature at which a solid substance changes into a liquid state. It is a specific characteristic of each substance and remains constant under normal conditions. The melting point can provide information about the purity and identity of a substance.
- Boiling Point: Boiling point is the temperature at which a liquid substance changes into a gaseous state. Like the melting point, it is a unique property of a substance and can help identify and characterize it.
- Conductivity: Conductivity refers to the ability of a substance to conduct heat or electricity. Some substances, such as metals, are good conductors, while others, like non-metals, are poor conductors (insulators). Conductivity depends on factors such as the mobility of charged particles (ions or electrons) within the material.
- Solubility: Solubility is the ability of a substance to dissolve in a solvent and form a homogeneous mixture. It indicates the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature. Solubility varies depending on the nature of the substance and the solvent.
These physical characteristics play a crucial role in identifying, classifying, and understanding matter. They provide valuable information about the properties and behavior of materials in various scientific, industrial, and everyday contexts.
Molecular Theory of Matter
The molecular theory of matter is a scientific concept that explains the behavior and properties of matter based on the arrangement and motion of its constituent particles, such as atoms, molecules, and ions. It provides a framework for understanding how matter behaves in different states.
According to the molecular theory:
- Matter consists of small particles, such as atoms, molecules, or ions, that are constantly in motion.
- Particles in matter are not packed tightly together. They have space between them, even in solids.
- Particles are in a state of continuous motion. The magnitude and nature of their motion depend on the state of matter.
- Particles interact with each other through forces such as intermolecular forces or electrostatic forces. These interactions determine the physical and chemical properties of matter.
The Solid State of Matter
The solid state of matter is one of the four fundamental states of matter, along with liquid, gas, and plasma. Solids are characterized by their rigid structure, definite shape, and resistance to deformation. In this state, the particles that make up the substance are closely packed and arranged in a regular pattern, forming a three-dimensional lattice.
Some Key Characteristics and Properties of Solids
- Particle Arrangement: In a solid, the particles, which can be atoms, molecules, or ions, are tightly packed and held together by strong intermolecular or intramolecular forces. The arrangement of particles can be crystalline or amorphous.
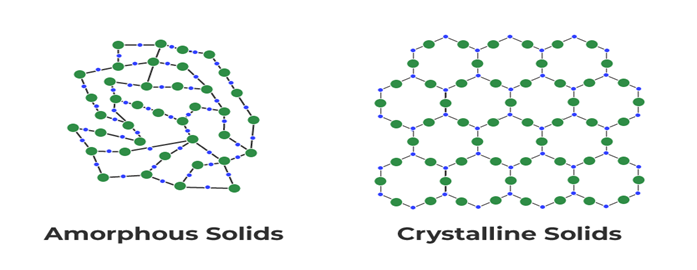
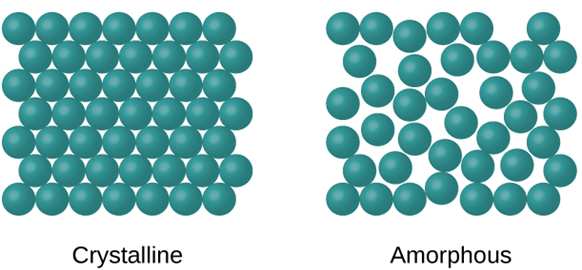
Crystalline Solids: Crystalline solids have a highly ordered arrangement of particles, forming a repeating pattern called a crystal lattice. Examples include salt (sodium chloride), diamond, and quartz.
Amorphous Solids: Amorphous solids lack a regular and repeating arrangement of particles. Their structure is more disordered. Examples include glass, rubber, and some plastics.
- Definite Shape and Volume: Solids have a fixed shape and volume. The strong forces between particles prevent them from freely moving around. They vibrate in fixed positions, but their overall arrangement remains intact.
- Density: Solids generally have a higher density compared to liquids and gases because of the close packing of particles.
- Melting Point: Solids have a specific melting point, which is the temperature at which they transition into the liquid state. At this temperature, the thermal energy overcomes the intermolecular forces holding the particles together, causing them to break free and enter a more disordered state.
- Mechanical Strength: Solids are generally strong and rigid due to the strong intermolecular or intramolecular forces. This gives them the ability to resist deformation and maintain their shape.
- Thermal Expansion: Solids expand when heated and contract when cooled. This property is used in various applications, such as in the construction of bridges and buildings, where materials need to accommodate temperature changes without structural damage.
- Electrical and Thermal Conductivity: The conductivity of solids varies depending on their composition and structure. Some solids, such as metals, conduct electricity and heat well due to the presence of mobile electrons. Others, like most non-metals, are poor conductors.
- Optical Properties: Solids exhibit a range of optical properties. They can be transparent, translucent, or opaque, depending on their molecular structure and interactions with light.
It’s worth noting that while these characteristics generally apply to solids, there can be variations depending on the specific substance and its atomic or molecular composition.
Overall, the solid state of matter plays a vital role in various aspects of our everyday life, from the materials we use to the structures we build, making it an essential area of study in physics and materials science.
The Liquid State of Matter
Liquids are characterized by their ability to flow and take the shape of their container. In this state, the particles that make up the substance have more freedom of movement compared to solids, but less than gases.
Here are some key characteristics and properties of liquids:
- Particle Arrangement: In a liquid, the particles, which can be atoms, molecules, or ions, are close together but not as tightly packed as in solids. The arrangement of particles in a liquid is less ordered and more random compared to the regular lattice structure of solids.
- Shape and Volume: Liquids take the shape of their container and do not have a definite shape of their own. However, they have a fixed volume, meaning they have a specific amount of substance that remains constant unless acted upon by external factors.
- Fluidity and Flow: Liquids have the ability to flow and move freely due to the moderate strength of intermolecular forces. The particles can slide past each other, allowing liquids to take the shape of their container and adapt to its contours.
- Density: Liquids have a higher density compared to gases but generally lower density than solids. The density of a liquid depends on the substance and its temperature.
- Surface Tension: Liquids exhibit surface tension, which is a property that allows liquids to minimize their surface area. It arises due to cohesive forces between liquid particles at the surface. This property gives rise to phenomena like droplet formation and capillary action.
- Viscosity: Viscosity refers to the resistance of a liquid to flow. Liquids with high viscosity flow slowly, while liquids with low viscosity flow more easily. Viscosity depends on factors such as temperature and molecular interactions.
- Vaporization: Liquids can undergo vaporization, transitioning into the gas state. This occurs through two processes:
Evaporation: The gradual conversion of liquid to vapor at the surface, typically at lower temperatures.
Boiling: The rapid conversion of liquid to vapor throughout the bulk of the liquid when it reaches its boiling point.
- Thermal Expansion: Like solids, liquids expand when heated and contract when cooled. However, liquids generally exhibit greater expansion compared to solids.
- Conductivity: Liquids can conduct electricity to varying degrees depending on their composition. Some liquids, like aqueous solutions of electrolytes, can conduct electricity, while others, like pure water or organic solvents, are poor conductors.
- Mixing and Dissolving: Liquids have the ability to mix and dissolve with other liquids or soluble substances, creating solutions.
The liquid state of matter finds numerous applications in various fields, such as in transportation, chemical reactions, and everyday activities like drinking water, cooking, and cleaning.
The Gaseous State of Matter
Gases are characterized by their ability to fill the entire volume of a container, expand to occupy any available space, and lack a definite shape or volume. In this state, the particles that make up the substance are far apart and move freely and rapidly.
Here are some key characteristics and properties of gases:
- Particle Arrangement: In a gas, the particles, which can be atoms or molecules, are widely spaced and move independently of each other. They are not held together by strong intermolecular forces, resulting in a lack of ordered arrangement.
- Shape and Volume: Gases do not have a definite shape or volume. They uniformly spread out to fill the entire container they occupy, taking its shape.
- Fluidity and Compressibility: Gases are highly fluid and can easily flow and mix with one another. They can be compressed to occupy a smaller volume by applying pressure. This is due to the large gaps between the particles.
- Density: Gases have a lower density compared to liquids and solids. The density of a gas depends on factors such as temperature, pressure, and the molecular mass of the gas.
- Diffusion and Effusion: Gases exhibit rapid diffusion, which is the spontaneous mixing of one gas with another. They also undergo effusion, which is the process of a gas passing through a tiny opening or porous barrier.
- Expansion: Gases expand to fill any available space uniformly. When heated, they expand further due to increased molecular motion and collisions.
- Pressure: Gases exert pressure on the walls of their container. Gas pressure is caused by the collisions of gas particles with the container walls. The pressure increases with an increase in the number of gas particles or their kinetic energy.
- Thermal Expansion: Gases expand significantly with an increase in temperature and contract when cooled. The relationship between temperature and volume is described by the gas laws, such as Charles’s Law and the Ideal Gas Law.
- Low Intermolecular Forces: Gaseous particles have weak intermolecular forces compared to liquids and solids. This allows them to move freely and independently of each other.
- Mixing and Effusion Rates: Gases mix spontaneously and rapidly due to their high molecular speeds. The rate of effusion, or the escape of gas through a small hole, depends on the molecular mass of the gas.
- Lack of Definite Boiling or Melting Point: Gases do not have a specific boiling or melting point. Instead, they undergo a continuous transition from the gas phase to the liquid or solid state when cooled or compressed sufficiently.
The gaseous state of matter is involved in various applications and phenomena, such as atmospheric processes, combustion, gas laws, weather patterns, and many industrial processes.
The Plasma State of Matter
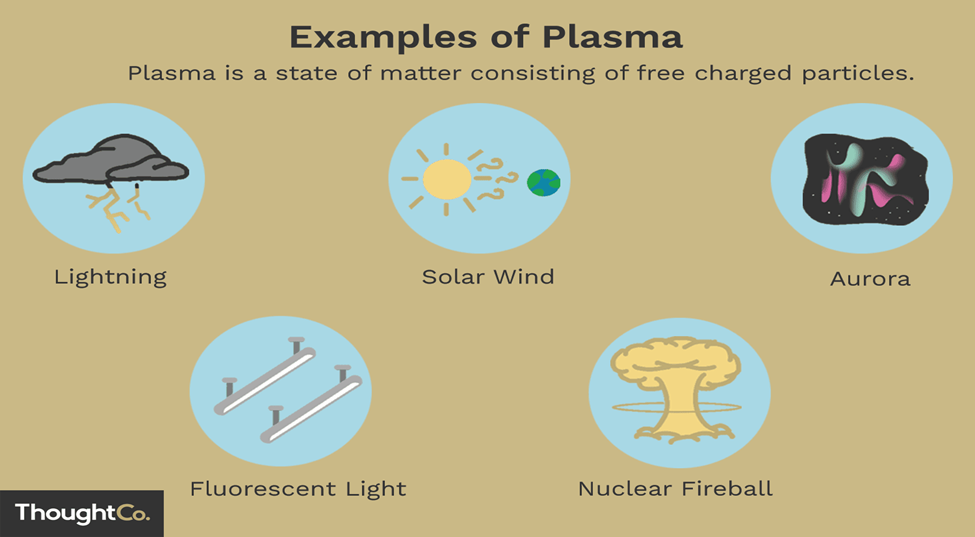
Here are some key characteristics and properties of plasma:
- Ionization: Plasma is formed when a gas is heated to extremely high temperatures or subjected to intense electromagnetic fields, causing the atoms or molecules to lose or gain electrons, thus becoming ionized. The presence of free-moving charged particles distinguishes plasma from other states of matter.
- Electrically Conductive: Plasma is an excellent conductor of electricity due to the presence of free-moving charged particles. It can carry electric currents and respond to electric and magnetic fields. This property is crucial in applications such as plasma TVs, fusion reactors, and fluorescent lights.
- Emission of Light: Plasma emits light due to the excitation and de-excitation of electrons. This emission can range from visible light to ultraviolet and even X-rays, depending on the temperature and composition of the plasma. Examples of plasma emitting light include neon signs, auroras, and stars.
- High Energy: Plasma contains a significant amount of thermal energy, as it is usually formed at very high temperatures. This energy can be harnessed for various applications, such as plasma cutting, welding, and thermonuclear fusion research.
- Non-Neutral: Unlike neutral gases, plasma has an overall electrical charge due to the presence of ions and electrons. The behavior of plasma is affected by electric and magnetic fields, and it can generate its own fields through the movement of charged particles.
- Plasma Waves and Instabilities: Plasma can support a wide range of collective wave-like behaviors, including plasma waves and instabilities. These phenomena arise due to the interactions between charged particles and electromagnetic fields and play a crucial role in plasma physics and fusion research.
- Variety of States: Plasma can exist in different states depending on the temperature and density. Some notable plasma states include fully ionized plasmas, partially ionized plasmas, and non-equilibrium plasmas, each exhibiting distinct properties and behaviors.
- Complex Dynamics: Plasma exhibits complex dynamics and interactions due to the collective behavior of charged particles. These dynamics give rise to phenomena such as plasma turbulence, magnetic reconnection, and plasma oscillations.
Plasma finds numerous applications, ranging from everyday technologies like fluorescent lights and plasma TVs to advanced fields such as fusion energy research, plasma propulsion in space exploration, and plasma medicine. The study of plasma physics is vital for understanding astrophysical processes, laboratory plasma experiments, and developing cutting-edge technologies.
Molecular Explanation for States of Matter
Molecular Explanation for Solid
In the solid state of matter, the molecular explanation focuses on the arrangement and behavior of particles, typically atoms or molecules, within a solid substance.
- In a solid, the particles are tightly packed and held together by strong intermolecular forces. The arrangement of particles is often in a regular, repeating pattern known as a crystalline structure. This ordered arrangement gives solids their definite shape and fixed volume.
- Despite being held in a fixed position, particles in solids still possess vibrational motion. They oscillate around their equilibrium positions due to thermal energy. This vibrational motion is limited to a small region, and the particles maintain relatively fixed positions relative to each other.
- The intermolecular forces, such as electrostatic forces or chemical bonds, are responsible for holding the particles together in a solid. These forces vary in strength depending on the nature of the substance. Ionic solids have strong electrostatic forces, while molecular solids have weaker forces between molecules.
- The arrangement of particles in a solid is often described by a lattice structure, which represents the repeating pattern of particles in a crystalline solid. Examples include cubic, tetragonal, and hexagonal lattices. The lattice structure determines the overall shape and symmetry of the solid.
- The density of a solid depends on the arrangement and packing of particles within the lattice structure. Some solids have close-packed arrangements, where particles occupy the maximum space available, resulting in higher densities. Others have less efficient packing, leading to lower densities.
- Solids expand when heated and contract when cooled. The expansion is due to the increase or decrease in the average vibrational amplitude of particles. However, solids generally have lower thermal expansion compared to liquids and gases.
- Solids exhibit elasticity, meaning they can deform under the application of external forces and return to their original shape when the force is removed. This behavior arises from the intermolecular forces that act as springs, allowing the solid to resist deformation.
- Some solids, particularly metals, exhibit high thermal conductivity. This is because particles in metals are closely packed and can transfer thermal energy efficiently through rapid vibration and collision of electrons and particles.
- In some solids, such as metals, the presence of free electrons allows for the flow of electric current. These solids are called conductors. Other solids, such as nonmetals, are poor conductors or insulators because their tightly bound electrons do not move as freely.
Understanding the molecular explanation of the solid state helps explain properties such as rigidity, brittleness, crystalline structures, and mechanical strength observed in various solid substances. The specific arrangement and behavior of particles determine the unique properties exhibited by different types of solids.
Molecular Explanation for Liquid
In the liquid state of matter, the molecular theory provides an explanation for the behavior and properties of liquids based on the arrangement and motion of their constituent particles, which are typically molecules.
Here is a molecular explanation for the liquid state:
- Molecular Arrangement: In liquids, the molecules are in close proximity to each other but are not rigidly fixed in a specific arrangement like in solids. The molecules have more freedom to move and are not held in fixed positions. However, they are still attracted to each other through intermolecular forces, which keep the liquid cohesive.
- Intermolecular Forces: Intermolecular forces, such as van der Waals forces, dipole-dipole interactions, and hydrogen bonding, play a crucial role in the behavior of liquids. These forces arise due to the attractions and repulsions between the charged or polar ends of molecules. They are weaker than the covalent or ionic bonds within molecules but strong enough to keep the molecules relatively close together.
- Molecular Motion: The molecules in a liquid are in constant motion. They have kinetic energy that allows them to vibrate, rotate, and translate within the liquid. This molecular motion is influenced by the temperature of the liquid. As the temperature increases, the average kinetic energy of the molecules increases, resulting in faster and more energetic motion.
- Fluidity and Flow: The ability of liquids to flow and take the shape of their containers is attributed to the relative freedom of molecular motion. The molecules can slide past each other, allowing liquids to exhibit fluidity and adapt to the container’s contours. However, the intermolecular forces still keep the molecules in close proximity, providing some resistance to flow.
- Density and Compressibility: Liquids have higher density compared to gases but lower density than solids. This is because the molecules in a liquid are closer together compared to gases but not as densely packed as in solids. Liquids are generally not easily compressible due to the close proximity of the molecules and the intermolecular forces between them.
- Surface Tension: Liquids exhibit surface tension, which is the result of the cohesive forces between the molecules at the surface of the liquid. Molecules within the liquid experience attractive forces in all directions, but those at the surface experience a net inward force. This gives rise to a phenomenon where liquids tend to minimize their surface area, forming droplets or displaying capillary action.
The molecular explanation for the liquid state provides insights into various properties and behaviors of liquids, including their ability to flow, adapt to containers, and exhibit surface tension. Understanding the molecular interactions in liquids is crucial in many scientific fields, such as chemistry, physics, biology, and engineering.
Molecular Explanation for Gas
The gaseous state of matter can be explained at the molecular level using the kinetic molecular theory.
According to this theory:
- Particle Motion: In the gaseous state, individual gas particles, which can be atoms or molecules, are in constant, rapid, and random motion. They move in straight lines until they collide with other particles or the walls of the container.
- Particle Spacing: Gas particles are widely spaced compared to liquids and solids. The average distance between gas particles is much greater than their own size. As a result, the volume occupied by gas particles themselves is negligible compared to the total volume of the gas.
- Particle Energy: Gas particles possess kinetic energy due to their motion. The kinetic energy is directly related to the temperature of the gas. The higher the temperature, the greater the average kinetic energy of the particles.
- Particle Collisions: Gas particles undergo frequent collisions with each other and with the walls of the container. These collisions are perfectly elastic, meaning that there is no net loss of kinetic energy during the collisions.
- Forces Between Particles: In the gaseous state, the intermolecular forces between gas particles are relatively weak. These forces are primarily attractive van der Waals forces or dipole-dipole interactions, which are much weaker compared to the strong forces present in solids and liquids.
- Expansion and Contraction: Gases have the ability to expand and fill the entire volume of the container they are placed in. When the volume of the container is increased, the gas particles have more space to move and spread out. Conversely, when the volume is decreased, the gas particles become more crowded, leading to an increase in pressure.
- Pressure: The pressure of a gas is a measure of the force exerted by the gas particles on the walls of the container per unit area. Pressure arises due to the collisions of gas particles with each other and with the container walls. The more frequent and energetic the collisions, the higher the pressure.
The molecular explanation of the gaseous state provides insights into the macroscopic properties of gases, such as their ability to expand, fill containers, and exert pressure. It helps explain gas laws, such as Boyle’s Law, Charles’s Law, and the Ideal Gas Law, which describe the relationship between pressure, volume, temperature, and number of gas particles.
In the plasma state of matter, the behavior and properties are explained at the molecular level by considering the presence of highly energized and ionized particles. Plasma is often referred to as the fourth state of matter and is characterized by the presence of free electrons and ions.
Molecular Explanation for Plasma
In a plasma, the majority of atoms or molecules are stripped of their electrons, resulting in a mixture of positive ions and negatively charged electrons. This ionization occurs when sufficient energy is supplied to the gas, typically through high temperatures or strong electromagnetic fields.
The molecular explanation for the plasma state involves the following key aspects:
- Ionization: In a plasma, the high energy of the particles causes the electrons to be separated from their parent atoms or molecules, resulting in positively charged ions and free electrons. The energy provided to the gas can be thermal energy, electric fields, or other energy sources.
- Electrically Charged Particles: The presence of ions and electrons in plasma leads to electrical conductivity. The positive and negative charges allow plasma to respond to electric and magnetic fields and carry electric currents.
- Collective Behavior: Plasma exhibits collective behavior due to the interactions between the charged particles. Electromagnetic forces influence the motion of the charged particles, leading to phenomena like plasma waves and instabilities.
- Debye Shielding: In a plasma, the free electrons and positive ions interact with each other through Coulombic forces. This interaction creates a shielding effect called Debye shielding, where the negative charges surrounding a positive ion partially neutralize its electric field.
- Electrically Charged Particles: The presence of ions and electrons in plasma leads to electrical conductivity. The positive and negative charges allow plasma to respond to electric and magnetic fields and carry electric currents.
- Collective Behavior: Plasma exhibits collective behavior due to the interactions between the charged particles. Electromagnetic forces influence the motion of the charged particles, leading to phenomena like plasma waves and instabilities.
- Debye Shielding: In a plasma, the free electrons and positive ions interact with each other through Coulombic forces. This interaction creates a shielding effect called Debye shielding, where the negative charges surrounding a positive ion partially neutralize its electric field.
- Plasma Oscillations: The charged particles in a plasma can undergo oscillatory motion, giving rise to plasma oscillations or plasma waves. These oscillations can be longitudinal or transverse, similar to electromagnetic waves, and play a significant role in plasma physics.
- Collisional Processes: Collisions between particles in a plasma occur frequently due to their high kinetic energies. These collisions determine the thermalization of energy and can lead to energy transfer, excitation, and ionization of particles.
- Plasma Equilibrium: Despite the presence of charged particles, a plasma can reach a state of equilibrium. In this state, the rates of ionization, recombination, and other collisional processes reach a balance, resulting in a steady distribution of charged particles.
The molecular explanation of plasma provides insight into the behavior and properties of this state of matter. It helps understand phenomena such as plasma confinement in fusion reactors, the generation of plasma in stars and lightning, and the applications of plasma in technologies such as plasma TVs, semiconductor processing, and plasma propulsion.